Impressed current cathodic protection is a common technique for shielding metal structures against galvanic deterioration. This blog post discusses how the method works and for which applications it is generally used. An example of such simulations on a ship hull is revealed, and the critical points for application are explained. In this design, among the essential facets is the electrical field signature that arises from the shaft’s cathodic protection system and the propeller onboard.
The Corrosion Problem
Corrosion of metals is a naturally occurring electrochemical process that causes the metal to oxidize and deteriorate when exposed to the environment (commonly referred to as rusting.) Cathodic protection (CP) is a means to prevent corrosion by applying a flow of electrical current from an external source (anode) through the environment and on to the metallic structure that is being protected. This protective current changes the environment around the metal thus halting the corrosion reaction. When properly designed and applied, cathodic protection systems stop the corrosion process. Cathodic protection is used to prevent corrosion in a wide range of applications where the structure being protected is surrounded by an environment that allows current flow. Unfortunately, atmospherically exposed metal cannot be cathodically protected because air is not a conductor of electrical current, but most submerged and buried applications are suitable for cathodic protection including pipelines, ships, docks, jetties, storage tanks, and a range of other structures.
Basic Types of Cathodic Protection
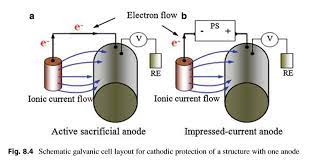
There are two basic types of CP systems: galvanic (or sacrificial) and impressed current. Galvanic anode systems (also termed sacrificial anode systems) use a metal that is naturally more negative than the metal being protected and thus when the two metals are connected electrically to each other, current flows from the metal that is more electrically negative to the metal that is more electrically positive. This current flow results in a rapid consumption of the anode, hence the common term “sacrificial anode” is often used to describe these anode systems. Typical galvanic anodes include magnesium, zinc and aluminum as each of these are more electrically negative than carbon steel or other steels.
Impressed current anode systems are different than galvanic (sacrificial) systems because they utilize an external DC power supply to create the electrical current flow. The use of an external power supply enables an impressed current system to generate significantly higher current output with fewer, longer lasting anodes than any sacrificial anode system.
Why Impressed Current Cathodic Protection (ICCP)?
- Longer Life Anodes. Because the choice of anodes is not dependent on the electrical potential of the anode itself, impressed current anodes can be selected based on other factors such as anode material cost, current density and consumption rates. Today’s most efficient anodes are the dimensionally stable mixed metal oxide anodes (MMO.) These anodes have exceptionally long anode life relative to other anode choices.
- Higher Current Systems. The basic formula for a DC circuit is that V = I x R (Ohm’s law) where V is the voltage difference or driving force, I is the system current and R is the system resistance. The system resistance is in large part dictated by the environment and not easily changed. For galvanic anodes the value of V is fixed and less than 1 Volt thus the amount of current that can be generated from a galvanic anode is also limited. With ICCP systems, the driving voltage is a function of the size of the cp rectifier and typical voltages can range from 20V to 100V. Much greater than that of any galvanic system.
- Greater System Control. With a galvanic anode system, there is only a very limited amount of ways to control the system output. For most galvanic systems they are installed and left to operate with no controls in place. In some cases they work as designed, and in other cases they either operate too fast causing premature failure or they do not operate sufficiently to protect the structure. But once installed you have little control over their operation. With an impressed current system, the power supply can be adjusted to change the current being supplied to the anode system. If too much current is being discharged the system power supply output can be turned down, of conversely if the current being supplied is not sufficient, then the system output can be increased (at least up to the limit of the power supply being used.)
- Ease of Monitoring and Control. With an impressed current system, the power supply provides an easy means of monitoring and controlling the system performance. Once installed and properly commissioned, the impressed current cathodic protection system rectifier provides an easily verifiable monitoring point. Monthly readings of the power supply output voltage and current can be checked to assure consistency with previous readings. With a galvanic anode system, specialized provisions for testing are required in the design to confirm the system performance.
MATCOR Impressed Current Cathodic Protection Systems
MATCOR is a leader in the design, manufacture and installation of impressed current cathodic protection systems, including:
- SPL™-Anode Series Impressed Current Linear Anodes
- Durammo® Deep Anode System
- MMP-Anode Prepackaged MMO Impressed Current Anode
Contact us at the link below to find out if an impressive current cathodic protection system is right for your application.